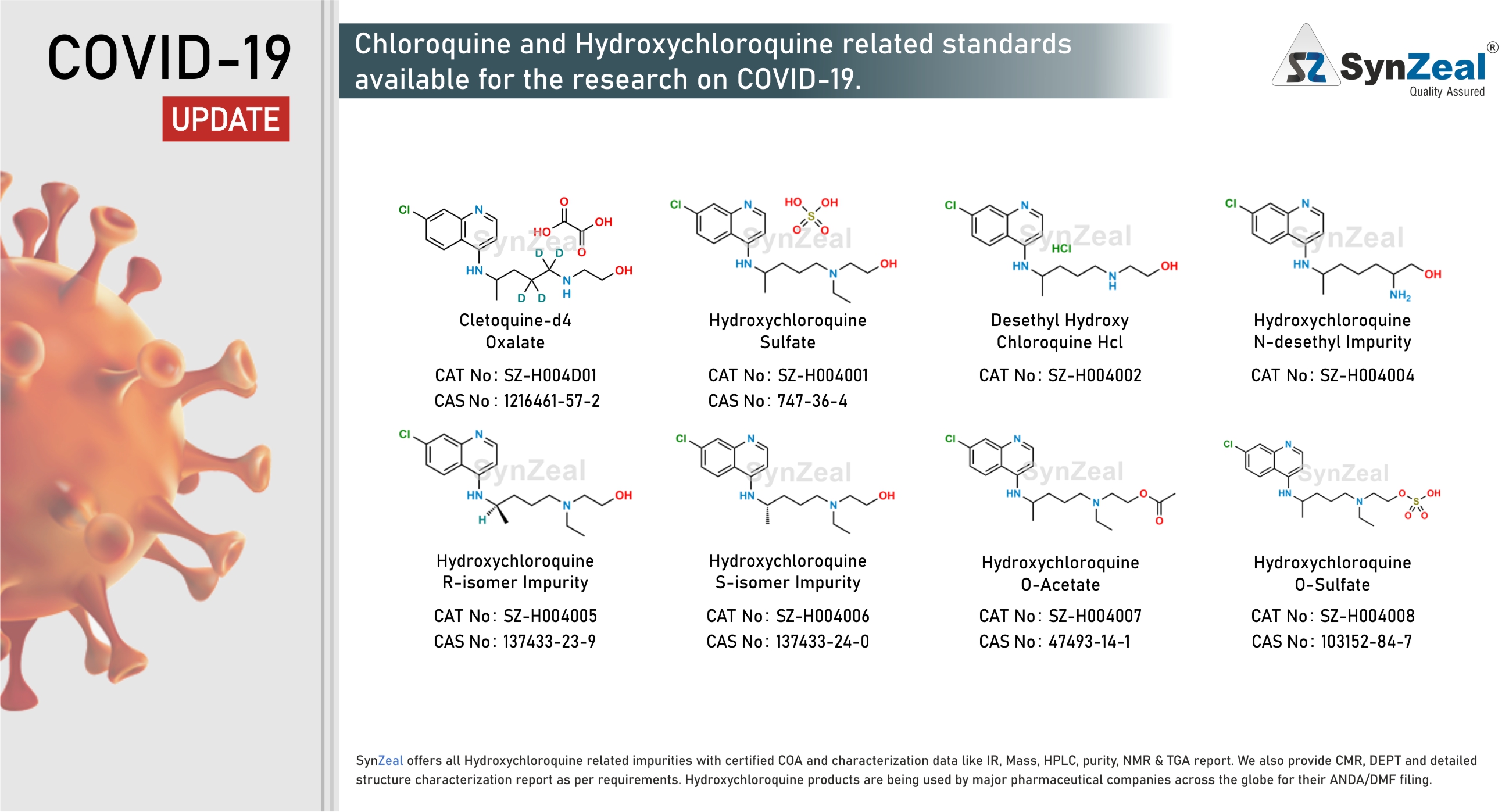
Hydroxychloroquine # The antimalarial drug chloroquine and its safer derivative hydroxychloroquine are commonly prescribed medication in the treatment of uncomplicated malaria, rheumatoid arthritis, chronic discoid lupus erythematosus, and systemic lupus erythematosus. These drugs have also attracted attention over the past few decades as a potential antiviral agent, currently as a possible treatment for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19. Reports found that chloroquine could inhibit SARS-CoV-2 in vitro, and showed apparent efficacy in treating COVID-19 in humans. The US Food and Drug Administration has designated hydroxychloroquine for off-label, compassionate use for treating COVID-19, and WHO added the drug to its large global solidarity trial to test a variety of potential treatments.
Ritonavir & Lopinavir # Currently, there are no drugs documented to be effective to treat patients with COVID-19 arising from coronavirus SARS-CoV-2 infection. Without an approved drug to tackle the novel coronavirus, pharmaceutical companies are looking to repurpose existing therapies. Lopinavir/Ritonavir medication is widely used for the treatment and prevention of HIV and AIDS. Lopinavir, which acts against the viral 3CL protease, has also modest antiviral activity against SARS-CoV-2. Together with ritonavir, which increases drug bioavailability, it is in clinical trials, along with the immunomodulator interferon beta-1b.
Remdesivir # When COVID-19 began its march across the world, so did a desperate hunt for a treatment. Gilead Sciences Inc’s Remdesivir, or GS-5734, is an adenosine triphosphate analog first described in the literature in 2016 as a potential treatment for Ebola. Remdesivir is a nucleoside analogue prodrug, has inhibitory effects on human coronaviruses. Recently US FDA issued EUA of Remdesivir and now Japan also approved Remdesivir as a treatment for COVID-19, making it the country's first officially authorised drug to tackle the coronavirus disease.
SynZeal offers all Hydroxychloroquine, Lopinavir, Ritonavir, Remdesivir related impurities with certified COA and characterization data like IR, Mass, HPLC, purity, NMR & TGA report. We also provide CMR, DEPT and detailed structure characterization report as per requirements. Hydroxychloroquine, Lopinavir, Ritonavir, Remdesivir products are being used by major pharmaceutical companies across the globe for their ANDA/DMF filing.




