OUR BLOGS
Check out our latest and upcoming Blogs.
Contact Us.png)
April 28 2022
Fluocinolone binds the glucocorticoid receptor, followed by translocation of the ligand-receptor complex to the nucleus and transcription activation of genes containing glucocorticoid-respons...
Read More ›
April 22 2022
Fesoterodine also known as toviaz, belongs to the class of organic compounds known as diphenylmethanes. Fesoterodine is a competitive muscarinic receptor antagonist with muscle relaxant and uri...
Read More ›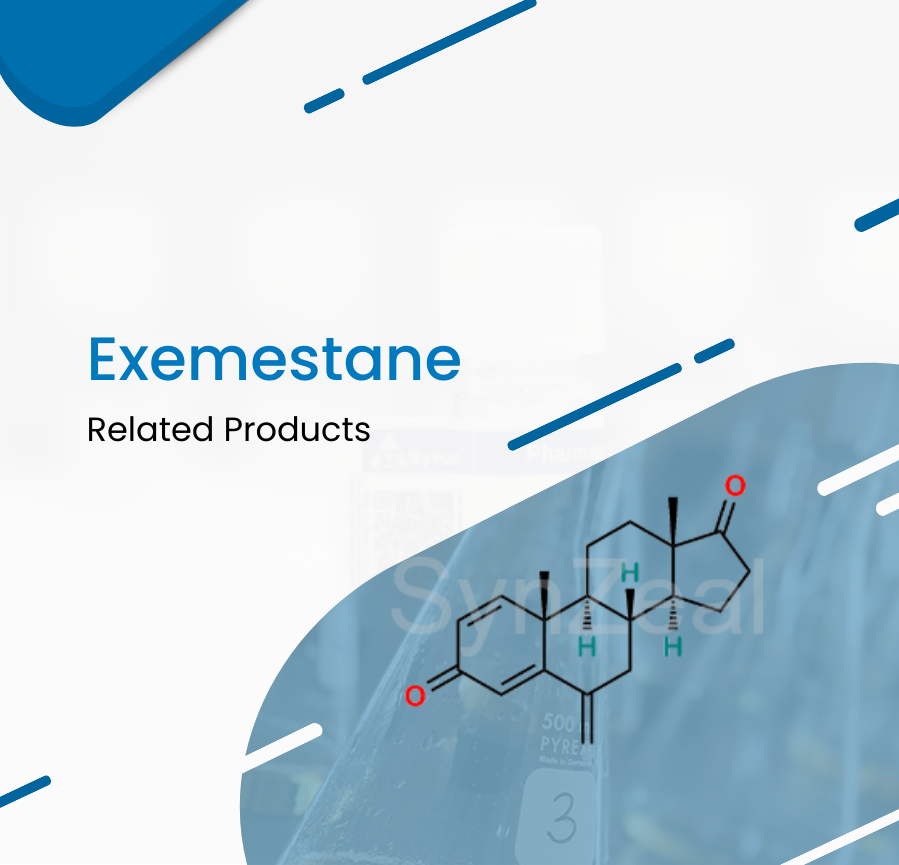
April 14 2022
Exemestane binds irreversibly to and inhibits the enzyme aromatase, thereby blocking the conversion of cholesterol to pregnenolone and the peripheral aromatization of androgenic precursors into...
Read More ›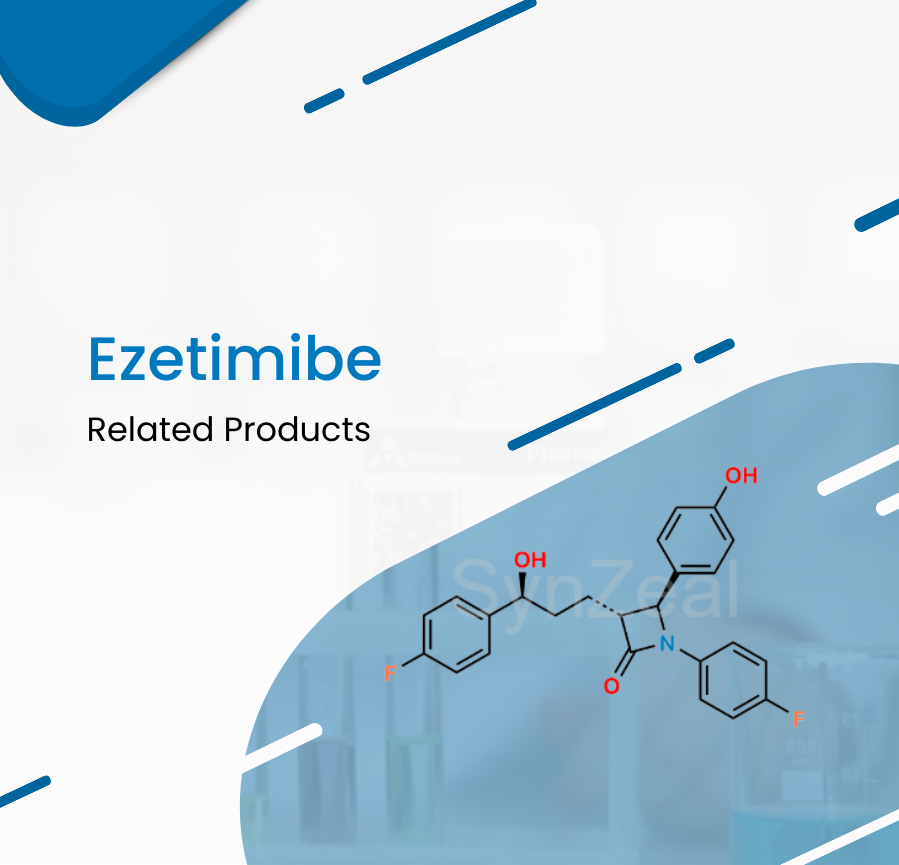
April 8 2022
Ezetimibe is a lipid-lowering compound that inhibits intestinal cholesterol and phytosterol absorption. Ezetimibe is used as an adjunctive therapy to a healthy diet to lower cholesterol levels in prim...
Read More ›
March 31 2022
Etonogestrel molecule is a 3-ketodesogestrel or 19-nortestosterone which is a synthetic biologically active metabolite of progestin desogestrel.Etonogestrel is a drug which is used for use as a female...
Read More ›